| Hyun Jung Kim | 2 Articles |
Background
In the case of clinical practice guideline (CPG), the need for the prospective registration of protocols has been proposed several times. However, the registration of CPG protocols is not yet active. The objective of this study was to summarize the experience of the CPG protocol registration program in Korea. Methods This study was performed in the following order: 1) formation of a methodological expert group; 2) CPG protocol template development; 3) CPG protocol preparation and expert review; 4) exploration of the knowledge and attitude of the guideline developers toward CPG protocol. Results The final version of the CPG protocol templates consists of four parts (planning, development, finalization, and timetable). The protocols for 18 cancers were submitted by 14 medical societies. conflicts of interest (n = 14, 77.8%), guideline development group (GDG; n = 9, 50%), scope of CPG (n = 9, 50%), and key questions (n = 8, 44.4%) were the under-reported areas in the submitted protocols. The GDGs (n = 13, 72.7%) was the most misreported areas of the protocol. CPG developers generally agreed on the advantages of protocol registration but responded that it was difficult to understand the concepts in the protocol and fill them with appropriate content. The areas where CPG developers responded that they felt difficulty were were recommendation grade (n = 9, 75.0%), GDG composition (n = 7, 58.3%), and determining key questions (n = 7, 58.3%). Conclusions The CPG protocol registration program was planned and piloted in Korea, and it could be said that it is feasible. It is necessary to evaluate the developed CPG later and determine whether protocol registration affects the quality of CPG through indices such as transparency and clarity of CPG.
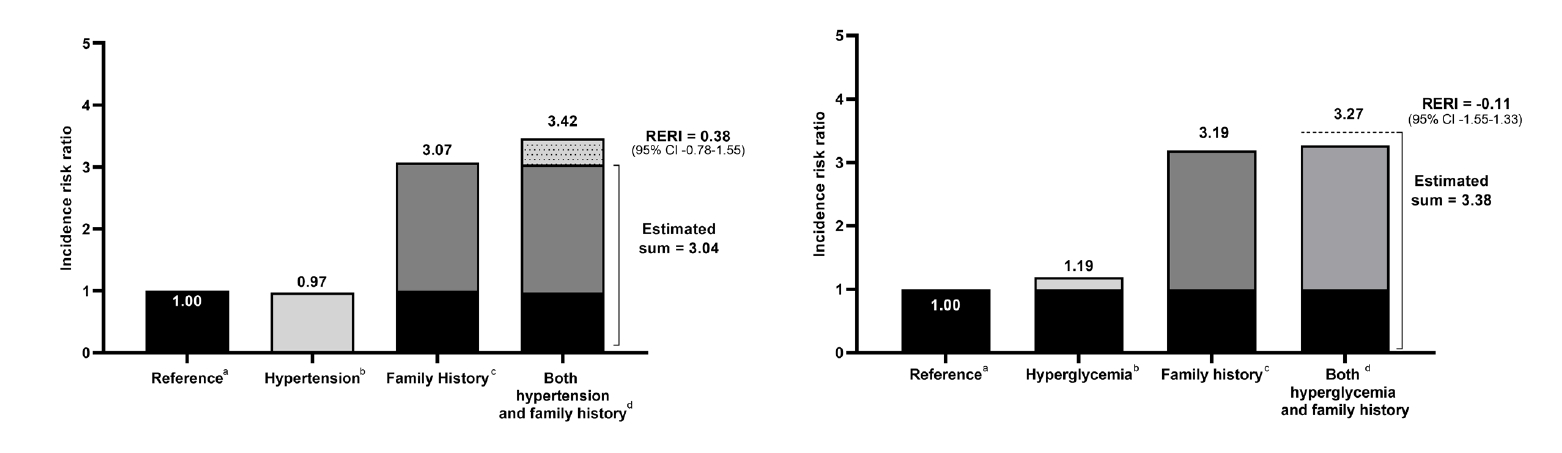
Although there is a genetic component to primary open-angle glaucoma (POAG) susceptibility, few studies have investigated interactions between genetic and environmental factors. We aimed to quantify the familial risk of POAG and estimate disease risk among individuals with a positive family history and either hypertension or hyperglycemia, as well as assess their interactions. Using the National Health Insurance database, which includes information on familial relationships and lifestyle risk factors, we identified 6,217,057 individuals with first-degree relatives (FDRs) from 2002-2018. We calculated familial risk using hazard ratios (HRs) with 95% confidence intervals (CIs) which compare the risk of individuals with and without affected FDRs. Disease risk was estimated among individuals with both a positive family history and hypertension or hyperglycemia, and interactions were assessed on an additive scale. Individuals with an affected parent had a 3.13-fold (95% CI 2.74 –3.58) increased risk of disease compared to those with unaffected parents. Individuals with affected father, mother, or both affected parents showed HRs (95% CI) of 3.50 (2.86 –4.30), 2.87 (2.41 –3.44) and 4.88 (1.83 –12.98), respectively. Familial risk adjusted for lifestyle factors decreased slightly (HR 3.14), suggesting that genetic component is the predominant driver in the familial aggregation. Individuals with a positive family history and either hypertension or hyperglycemia had a markedly elevated risk of disease, with HRs of 3.42 (95% CI 2.49 –4.69) and 3.27 (95% CI 2.15 –4.97), respectively. Hypertensive or hyperglycemic individuals with a positive family history may be considered a high-risk group and glaucoma screening may be considered.
|
|


